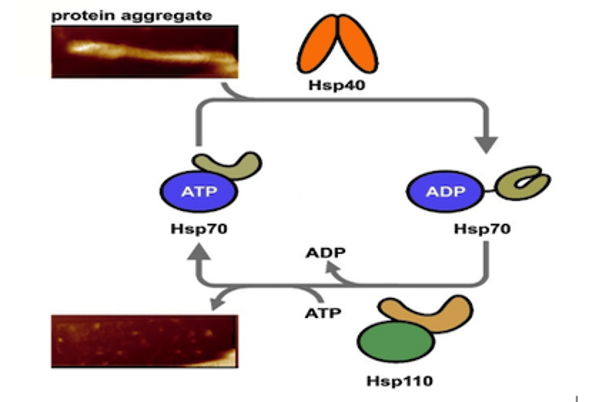
Protein Folding and Chaperones Laboratory

Universidad Autónoma de Madrid
Madrid, EspañaPublicaciones en colaboración con investigadores/as de Universidad Autónoma de Madrid (11)
2019
-
Structural insights into the ability of nucleoplasmin to assemble and chaperone histone octamers for DNA deposition
Scientific Reports, Vol. 9, Núm. 1
2010
-
Nucleoplasmin binds histone H2A-H2B dimers through its distal face
Journal of Biological Chemistry, Vol. 285, Núm. 44, pp. 33771-33778
2005
-
Ionic interactions at both inter-ring contact sites of GroEL are involved in transmission of the allosteric signal: A time-resolved infrared difference study
Protein Science, Vol. 14, Núm. 9, pp. 2267-2274
2003
-
GroEL stability and function. Contribution of the ionic interactions at the inter-ring contact sites
Journal of Biological Chemistry, Vol. 278, Núm. 34, pp. 32083-32090
2002
-
Purification and properties of TrwB, a hexameric, ATP-binding integral membrane protein essential for R388 plasmid conjugation
Journal of Biological Chemistry, Vol. 277, Núm. 48, pp. 46456-46462
2001
-
Excluded volume effects on the refolding and assembly of an oligomeric protein. GroEL, a case study
Journal of Biological Chemistry, Vol. 276, Núm. 2, pp. 957-964
1999
-
Characterization of ATP and DNA binding activities of TrwB, the coupling protein essential in plasmid R388 conjugation
Journal of Biological Chemistry, Vol. 274, Núm. 51, pp. 36117-36124
-
Conformational changes generated in GroEL during ATP hydrolysis as seen by time-resolved infrared spectroscopy
Journal of Biological Chemistry, Vol. 274, Núm. 9, pp. 5508-5513
1998
-
GroEL under heat-shock: Switching from a folding to a storing function
Journal of Biological Chemistry, Vol. 273, Núm. 49, pp. 32587-32594
1997
-
Effects of the inter-ring communication in GroEL structural and functional asymmetry
Journal of Biological Chemistry, Vol. 272, Núm. 52, pp. 32925-32932
1995
-
Prediction of the structure of GroES and its interaction with GroEL
Proteins: Structure, Function and Genetics, Vol. 22, Núm. 3, pp. 199-209

